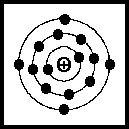

Fig 3.1. The silicon atom
A simplified representation of a silicon atom is shown in Fig 3.1. Around the positive nucleus there are three rings (orbits or shells) containing negatively charged electrons. The sum of the negative charges balances the positive charge of the nucleus - thus the atom is electrically neutral. The first ring contains two electrons and cannot accept any more. The next ring contains eight electrons. However, the third ring contains only four electrons and these join with the four electrons in the outer rings of adjacent atoms to form a crystal lattice. The outer electrons are not very far away from the nucleus and are not free to move from the lattice. Thus pure silicon is a good insulator.